
Understanding a few basics about physics for scuba diving will keep you safe underwater. This includes Boyle’s Law
Don’t let the thought of having to learn complicated physics or ‘scuba diving gas laws‘ stop you from learning how to scuba dive. All you really need to understand is some of the basic concepts. I aim to explain Boyle’s Law in relation to scuba diving in simple terms.
Boyle’s Law scuba diving in simple terms means the volume of gases in a diver’s body cavities and flexible spaces in their diving equipment decrease in size as they descend and water pressure increases. But in reverse these same air spaces increase in size as the scuba diver returns to the surface.
According to Wikipedia a modern statement of Boyle’s law is: The absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the temperature and amount of gas remain unchanged within a closed system.
The best way to do more diving is to book yourself on a scuba diving liveaboard. You can check the latest and best deals on liveaboards using the following window:
What is Boyle’s Law for Dummies and
how does Boyle’s law work?
Whilst I’m not a big fan of using the term ‘dummies‘. But this term is a recognised term for many and refers to the ‘basics of learning’. In other words the teaching or in this case my article on Boyle’s Law Scuba diving, is explained in simple terms. Well I hope so!
I hope I’ve achieved this. But if not, please pop a comment below and tell me your thoughts.
How does Boyle’s law work?
Boyle’s Law in scuba diving explains how the volume of gas varies with the surrounding water pressure. For the purpose of scuba diving the main gas that varies is air and nitrogen.
Understanding this simple ‘gas law‘ is key to staying safe when scuba diving. Take a moment to grasp this basic concept. Make sure you always follow the rules where Boyle’s Law affects you as a scuba diver. And as a result you shouldn’t encounter too many problems.
Before I get to how Boyle’s Law works in more detail, there’s a few concepts that need to be explained first. This begins with how the pressure of water changes with depth. But also about understanding that water is far more dense than air.

Boyle’s Law scuba diving concept #1 – Understanding that water is more dense than air
I think most understand that water is more dense than air. For example, air bubbles in water always rise to the surface, as seen in the above image with the scuba diver’s breathing exhaust bubbles.
This can also be explained using a simple experiment, as follows:
If you were to take two ‘empty’ buckets. Fill one bucket with water and leave the other bucket ‘empty.’ In fact the ’empty’ bucket isn’t actually empty, it’s full of air.
Now lift both buckets up with one in each hand.
If you didn’t realise this beforehand the heaviest bucket will be the one with water in it.
If you try this experiment you probably found it quite difficult to lift the water-filled bucket. Whereas the air-filled bucket would be easy to lift.
Even for those of you who already knew water is heavier than air, this simple explanation and experiment using two buckets highlights that water is clearly the heavier medium.
Water is nearly 800 times heavier than air at sea level
Water is nearly 800 (or 784 to be exact) times more dense than air at sea level. And the deeper you dive down the heavier water gets.
For example, at 10 metres (33 feet) water is at two bars of pressure or ‘two atmospheres‘. That’s twice the amount of pressure than you experience on land, where one atmosphere (or atmospheric pressure) is the pressure we experience at on land at sea level.
The first 10 metres of decent or the last 10 metres of ascent is where scuba divers experience the biggest pressure change.
Going deeper – the water around you gets heavier
The deeper you dive, the higher this pressure increases. For example:
- 20 metres (66 feet) water pressure is three bar or three times atmospheric pressure.
- 30 metres (100 feet) water pressure is four bar or three times atmospheric pressure.
- 40 metres (133 feet) water pressure is five bar or five times atmospheric pressure.
But as you ascend at the end of your dive the pressure reduces back down again. But why is water pressure important for scuba divers? And what has this got to do with Boyle’s Law and diver safety?
This is explained in Boyle’s Law scuba diving concept #2 below.
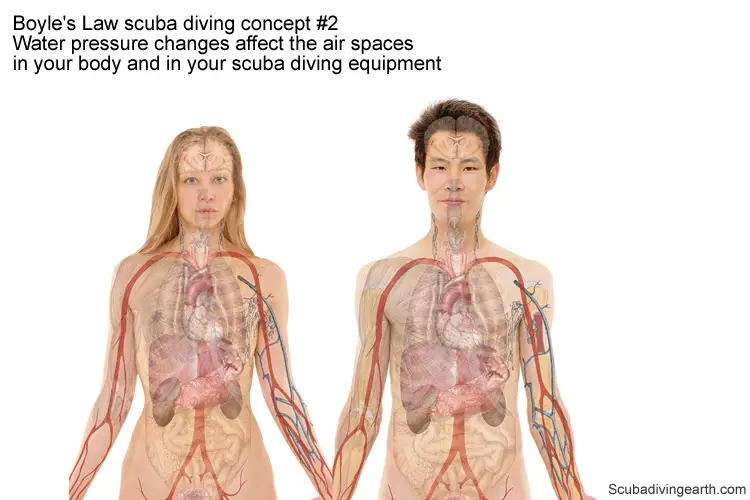
Boyle’s Law scuba diving concept #2 – Water pressure changes affect the air spaces in your body and in your scuba diving equipment
The second and very important concept to grasp is in how the pressure changes according to depth. Diving affects the air spaces in your body and in the flexible air spaces in your scuba diving equipment.
This is where the physics of Boyle’s Law is actually at play.
Keeping this very simple, Boyle’s Law is explained as follows:
- Assuming you have a flexible container filled with air. As the volume of this flexible container increases, the pressure of the air inside the contain decreases in proportion to the volume increase.
- Similarly, as volume of the flexible container decreases, the pressure of the air inside the container increases in proportion to the volume decrease.
Simple, right? If that is not clear to you, let’stake a look at an example.

Boyle’s Law scuba diving concept #3 – The Balloon experiment:
Firstly, the balloon represents any ‘flexible container filled with air‘. This flexible container might be your lungs or it could be your buoyancy control device.
If you fill a balloon with air at the surface and tie it off so that none of the air can escape. If you then take this balloon down to 10 metres (33 feet), it would halve in size. This is because the pressure at this depth of water has doubled, as explained above.
Even though the balloon has halved in size it still has the same amount of air inside, but the volume of the air inside the balloon has have halved.
Similarly, if you fill a balloon at 10 metres (33 feet) and tie it off so that none of the air can escape. Then bring this balloon to the surface, it would double in size.
Depending on how much air you put in the balloon at 10 metres, and depending on how flexible the balloon material is, will depend on whether the balloon could withstand this doubling in size. If it can’t then it would burst on its way to the surface.
However, on the assumption that the balloon doesn’t burst, but is now doubled in size, this means the volume of the air inside has increased proportionately. Which in this case the air volume inside the balloon will have doubled.
And that’s pretty much Boyle’s law for scuba diving explained. But for one additional element of the Boyle’s Law equation, which is how temperature affects the relationship between volume and pressure.
Boyle’s Law as it stands is stated where the temperature is a constant. In other words, temperature remains the same throughout the pressure changes.
In this video we will show you why you should never hold your breath while scuba diving. Lesson number one in your scuba diving course is to never hold your breath. And in this video we are going to show you what happens if you do hold your breath while scuba diving!
Changes in temperature affect Boyle’s Law
So how does temperature affect Boyle’s Law exactly?
In the above doubling of pressure or halving of pressure, the concept that the volume halves or doubles, assumes the temperature remains the same or constant during the descent or the ascent.
How does temperature affect Boyle’s Law?
If the temperature were to change during your descent or ascent, this will affect the amount of increase or decrease in the volume of air inside the balloon.
With scuba diving it’s quite likely that the temperature of the water could change. A temperature change could be brought about by passing through a thermocline. A thermocline is where the water temperature changes noticeably from one depth to another.
Having said that, and whilst temperature does affect the proportional changes of volume versus pressure, you don’t have to overly worry too much about this aspect of Boyle’s Law.
So long as you understand the basic principles or concepts described above, that’s all you’ll need to know to keep you safe. I’ve never thought about calculating temperature changes in relation to how this affects the changes in air spaces on my scuba equipment and in my body. All I know is that it happens.
Now that you hopefully understand more about Boyle’s Law in relation to scuba diving and underwater pressure changes, let me explain a bit more about the pressure of air. Air pressure changes when you fly could have an impact on you if you’ve been scuba diving beforehand, please read on.

Boyle’s Law scuba diving concept #4 – The pressure of air changes with altitude
You probably haven’t really given this much thought until now, but as a scuba diver it’s important to also understand that the pressure of air also changes. Air pressure changes with altitude.
This air-pressure change with air also impacts you and is also linked to Boyle’s Law. Let me explain further.
If you’ve ever flown before, you’ll be aware of the pressure change as you gain altitude in a plane. As the plane climbs in altitude, you will normally notice your ears ‘popping.’
This ‘ear popping’ is brought about by a reduction in air pressure. The changes in pressure are smaller with air and altitude than they are with water and depth. You have to change altitude significantly for a change to be noticed.
The pressure of air reduces with altitude
But nevertheless, air does get lighter as you go higher.
Another place where you may have experienced a large altitude change is driving into the mountains. This can be similar to flying.
More Reading: How long should you wait to fly after diving? (What’s safe?)
As you go higher into the mountain air pressure will reduce. In a similar way to flying you may experience ear popping too.
A safety recommendation for all scuba divers is to not fly or go to high altitude within 24 hours of their last scuba dive. This is because of these pressure changes, and as a result of Boyle’s Law in action.
In this instance the precaution is about decompression sickness. Decompression sickness is caused by small nitrogen bubbles forming in the blood and tissues of the body. This comes about as a result of the reducing air pressure in the case of flying. Please see below with regards to the bends and Boyle’s Law.
More Reading: What should you not do after scuba diving (11 must NOT do’s after diving)
Air spaces affected by Boyle’s Law scuba diving
There are a number of aspects relating to scuba diving that are affected by Boyle’s Law, which are as follows:

Breathing under water is affected by Boyle’s Law
Your lungs are flexible air spaces affected by Boyle’s Law. In fact your lungs behave in a very similar way to the balloon described above.
For example, if you fill your lungs with air on the surface and hold your breath and dive to 10 metres (33 feet), your lungs would halve in size. As a consequence the volume of the air in your lungs would halve too.
Similarly, if you fill your lungs with air at 10 metres (33 feet) with air from a dive tank and hold your breath (Note: never try this out, EVER) and ascended to the surface, the volume of air inside your lungs would attempt to double in size. But unlike the balloon experiment your lungs cannot double in size as they are surrounded by a rib cage.
Holding your breath at depth and ascending is sure to lead to rupture lungs
If your lungs were already at capacity at 10 metres, because you’ve taken a full breath, they are not likely to be able to double in size without rupturing. That’s not only because your lungs have a capacity, like a balloon, but is also because your lungs are protected by your rib cage. You rib cage limits your lung expansion.
It’s an absolute certainty that if you hold hold your breath with two full lungs of air from a dive tank at 10 metres and you ascend to the surface, your lungs would rupture. This is actually called a pulmonary barotrauma. A pulmonary barotrauma can also lead to air bubbles being released into the arterial circulation, which is known as an arterial gas embolism.
If you were to experience either a pulmonary barotrauma or a resulting arterial gas embolism, both are life threatening and should be avoided at all costs.
How does Boyle’s law affect breathing?
How Boyle’s law affects breathing underwater is possibly one of the most important rules to understand and follow as a scuba diver. As explained above, your lungs are similar to balloons because they are flexible air spaces made of a soft expandable material.
But even without the rib cage limitation and also like a balloon, your lungs have a capacity. After which they are likely to rupture. If you’ve ever blown up a balloon, but exceeded its maximum size, you’ll have experienced a loud bang as the balloon explodes. Your lungs are the same.
This is why it’s vitally important you breathe normally when you’re scuba diving. Which means you must never hold your breath.
More Reading: Why Is It Dangerous To Hold Your Breath While Scuba Diving?

The bends Boyle’s Law
How does Boyle’s Law relate to the bends?
“The bends“, which is also known as decompression sickness (DCS) occurs in scuba divers. This can happen either on an ascent from a dive or at high altitude if you fly too soon after scuba diving.
People have been known to ‘fizz-up’ on a plane because of flying too soon after scuba diving. The ‘fizzing‘ describes the formation of bubbles in the tissues and blood of the diver.
Decompression sickness that occurs after a dive when you ascend too fast or if you miss decompression stops on a decompression stop dive.
More Reading: How Deep Can You Dive Without Decompression (No Decompression Stop Limits)
The bends and Boyle’s Law are linked.
The bends (or decompression sickness) is caused when dissolved gasses (mainly nitrogen) come ‘out of solution‘ in the form of bubbles. This is the pressure/volume effect happening within the body. The gas, which in this case is nitrogen, changes in volume by forming bubbles, as the pressure reduces.
The bends is not just about underwater, but also can occur on land
The bends can affect just about any part of the body. This includes:
- The musculoskeletal system.
- Joints.
- Lungs.
- Heart.
- The skin.
- Your brain.
- The spinal cord.
Usual therapy for decompression sickness is recompression in a hyperbaric chamber. The diver is taken back down to depth using air pressure in the recompression chamber. This is so the volume of the bubbles are decreased and dissolved back into the body.
The diver suffering from decompression sickness is then decompressed very slowly. This slow decrease in pressure is so that the nitrogen gas has a chance to release from the body slowly without forming of bubbles.
Why can I hold my breath when I dive down when snorkeling?
You may be wondering why it is that the same doesn’t happen if you hold your breath when you dive down when snorkeling or in a swimming pool.
This is because you are breathing air in at the surface. As you descend to whatever depth you manage to reach as a snorkeler, the air and your lungs will compress with the pressure.
For example, if you were able to free-dive down to 10 metres (33 feet), the size of your lungs would halve in size under the pressure. Then as you rise back up to the surface, your lungs will return to their normal size.
The difference with scuba diving is you are breathing compressed air at depth.
How does Boyle’s law affect the human body and the other air spaces affect by pressure changes
There are other air spaces in addition to your lungs that are affected by the pressure changes underwater. These includes your middle ear and your sinuses.
Whilst the impact on these other air spaces is unlikely to be life threatening, nevertheless you need to be aware of them. This is particularly true of your middle ear.
The best way to avoid damaging your middle ear when scuba diving is to make sure you equalize the pressure. This can be done by performing a Valsalva manoeuvre (this is squeezing your nose and forcing air down your nostrils).
If you don’t equalise your ears the build up of air pressure inside your middle ear will lead to a burst eardrum.
With regards to your sinuses, there’s not a huge amount you can do to affect the changes in air volume inside your sinuses. However, your sinuses will be affected by having a cold. It is therefore not advisable to scuba dive when you have a cold or congestion.
This is because the congestion can block the Eustachian tubes at the back of your throat. This will prevent you from equalising the pressure, or actually correcting the air volume inside your middle ear.
Scuba diver buoyancy is affected by Boyle’s Law
Your buoyancy control device (BCD) is designed to work using the principles of Boyle’s Law. The main component of your buoyancy control device is the air bladder. This air bladder is also a flexible air space and is filled with air and helps you to maintain neutral buoyancy.
But if you change your depth, whilst at the same time not changing the amount of air in your BCD, the bladder of your BCD will either contract as you descend or expand as you ascend.
That is because the volume of air will change in proportion to the water pressure and the changes in depth.
BCD ascent control
When you ascend from a dive the water pressure decreases. As a result the volume of air in your BCD will expand. As the air expands your buoyancy will increase, which is why you need to release the excess air volume from your BCD as you ascend.
If you don’t release the expanding air you would lose control of your buoyancy. This would lead to a fast ascent and may result in decompression sickness.
BCD descent control
As you descend on a dive the water pressure increases. This means that the volume of air inside your BCD will reduce making you less buoyant. To correct your buoyancy you will need to add air to your buoyancy control device.
If you don’t add air to your BCD you would begin to sink like a stone.
Why is Boyle’s law so important to scuba divers?
Boyle’s Law is important because:
- It teaches you that holding your breath on an ascent from a dive is dangerous.
- Decompression sickness is the caused by the principles of Boyle’s law.
- Buoyancy control is affected by the Boyle’s law.
- It teaches you about the importance of ear equalisation.
What is the equation that represents Boyle’s Law?
If you are interested in the equation behind Boyle’s Law, this is what it looks like:
PV = k
What is the P in Boyle’s law?
The ‘P’ in Boyle’s Law represents pressure.
What is the V in Boyle’s law?
The ‘V’ in Boyle’s Law represents volume.
What is the K in Boyle’s law?
The ‘K’ in Boyle’s Law represents a constant value.
I hope you enjoyed this article about Boyle’s Law scuba diving
I’d love to hear from you. Tell us about your adventures of diving and snorkeling, in the comments below. Please also share your photos. Either from your underwater cameras or videos from your waterproof Gopro’s!
If this article hasn’t answered all of your questions. If you have more questions either about snorkeling or types of scuba diving (or specifically about Boyle’s Law scuba diving), please comment below with your questions.
There will also be many more articles about scuba diving (and snorkeling) for you to read and learn about these fabulous sports.
Have fun and be safe!





